What’s New: AHA 2025 Guideline Highlights for Mechanical CPR & LUCAS
The AHA’s release of its 2025 Guidelines for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiovascular Care (ECC) brings several important updates that users of mechanical CPR devices — including the LUCAS system — should know.
Here’s what these changes mean for first responders, hospitals, EMS services, and anyone involved in procuring or deploying automated/assisted CPR systems.
Key Guideline Changes Relevant to Mechanical CPR
1. Routine use of mechanical CPR devices is
not recommended
In the “Adult Basic Life Support” section, the AHA states that the “routine use of mechanical CPR devices is not recommended for adult cardiac arrest.”
What this means in practice:
Manual high-quality CPR remains the standard.
Mechanical devices should be reserved for specific circumstances where manual CPR is impractical, unsafe, or of poor quality.
EMS services and hospitals need to evaluate exactly when and how devices like LUCAS add value rather than assuming “automated is always better.”
2. Specified situations where mechanical CPR may be reasonable
While routine use is not the standard, there is acknowledgement that mechanical CPR devices may be reasonable in certain settings. For example:
During transport when it is difficult to maintain high-quality manual compressions (in ambulance, helicopter, or unusual terrain)
In situations where provider safety or physical conditions hamper effective manual compressions
In systems with protocols, training, and monitoring around mechanical CPR deployment
From commentary among EMS providers:
“The new guideline is that they are not recommended at all unless effective manual CPR cannot be performed, such as during transport (which itself is highly discouraged)…”
3. Integration into the “Chain of Survival” and system-of-care expectations
The 2025 Guidelines emphasize the importance of the unified Chain of Survival — prevention, recognition, high-quality CPR, defibrillation, advanced life support, post-arrest care — across in- and out-of-hospital settings.
This has implications for mechanical CPR device procurement and use:
Device deployment should be part of a system-wide plan, not as a standalone “silver bullet.”
Quality metrics (compression depth, rate, interruptions) remain critical whether manual or mechanical.
Training, protocols, debriefing, data capture (for both manual & mechanical) matter more than ever.
4. Implications for training, provider judgment & equipment procurement
The guidelines’ emphasis on context and provider judgment means:
Agencies must train crews not just on device operation, but on when not to use it versus when manual CPR should remain.
Procurement decisions should factor in systems of care, transport dynamics, terrain, manpower, quality monitoring and cost-benefit—not just device specs.
Post-event debriefing and review of mechanical CPR use will be more important to demonstrate that when LUCAS or similar devices are used, the decision was appropriate, supported, and audited.
What This Means for AEDPRO Customers & Partners
As a trusted partner in AED, CPR and resuscitation-equipment sales and service, AEDPRO is well positioned to help responder agencies interpret and implement these guideline changes.
For Purchasing / Equipment Decision-Makers
If your service is considering a mechanical CPR device (such as LUCAS), you’ll want to ask:
Under what specific scenarios will the device be deployed (transport, rescuer fatigue, space constraints)?
Is there a documented protocol that defines when manual compressions are insufficient?
Will you be monitoring quality metrics (compression depth, rate, hands-off time) and comparing manual vs device outcomes?
Has training been budgeted and is there a maintenance/service plan?
If you already have one, review usage data: Are you using it regularly? Were manual compressions of acceptable quality available? Does the device help in the scenarios you intended (e.g., difficult extrication, transport)?
Consider cost-effectiveness: With the guideline noting mechanical devices may be useful but are not routine, you’ll want ROI data: does the device reduce provider fatigue, improve hands-off time, support safety, enable earlier defibrillation, etc.?
For Training Coordinators & Medical Directors
Update your CPR protocols to align with the 2025 guidelines, explicitly stating the role (or limited role) of mechanical CPR.
Emphasize manual CPR quality — depth, rate, minimal interruptions, correct recoil — as the foundational standard.
Incorporate device use scenarios in simulation training: when to switch to device, how to deploy without delaying compressions, how to monitor for hands-off time during transition.
Capture data: time to device application, hands-off time during switch-over, outcomes when device used vs manual. Use these for continuous quality improvement.
For EMS / Fire / Hospital Staff
Recognize that mechanical CPR isn’t a replacement for solid manual compressions — device use must be appropriate and justified.
Be vigilant about minimizing delays/interruptions when deploying mechanical devices: frequent complaint is that switching to a device can create an extra pause.
Engage with debriefing and quality-review processes: when device was used, what went well, what didn’t, would the same situation have been managed just as well with manual CPR?
Understand equipment maintenance and readiness: even high-tech devices fail if not charged, serviced, cleaned, or integrated into the workflow.
Bottom Line for AEDPRO & Your Responder Community
The 2025 AHA Guidelines send a clear message: mechanical CPR devices like LUCAS are not the new default standard for all cardiac arrests. They can be very useful in specific circumstances — transport environments, where manual compressions are unsafe or ineffective, or when there are limited providers. But manual, high-quality CPR remains the bedrock of resuscitation.
For you, the responder agency or hospital — and for AEDPRO as your equipment/training partner — that means:
Make decisions on mechanical CPR devices thoughtfully, not reactively.
Build protocols, training, data capture and review systems that support when and how a device is used.
Emphasize manual CPR quality always; device use should enhance outcomes, not replace basics.
Use this guideline update as an opportunity to review your equipment inventory, training curriculum, deployment protocols, and service/maintenance agreements.
Sources:
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001369?utm_source=chatgpt.com
https://cpr.heart.org/en/resuscitation-science/cpr-and-ecc-guidelines/adult-basic-life-support
🧠 AI-Enabled Medical Devices: What the New FDA Guidance Means for 2025 and Beyond
The FDA is preparing new guidance for AI-enabled medical devices in 2025. Learn how these changes could impact AEDs, connected rescue systems, and emergency medical equipment buyers.
The Rise of Artificial Intelligence in Medical Devices
Artificial intelligence (AI) is no longer just for smartphones and self-driving cars. It’s entering the world of life-saving medical equipment — including automated external defibrillators (AEDs), vital-sign monitors, and hospital triage systems.
In early October 2025, the U.S. Food and Drug Administration (FDA) announced a new initiative seeking public input on how to evaluate and regulate AI-enabled medical devices. The move signals a major shift in how medical technology will be developed, tested, and approved over the next decade.
AI-enabled medical devices use algorithms to learn from data and improve over time — helping providers make faster, more accurate decisions in emergencies. Think of AEDs that can automatically analyze cardiac rhythms, predict the success of a shock, or even transmit real-time rescue data to emergency teams.
FDA’s 2025 Guidance: A Turning Point for AI in Healthcare
The FDA’s newest effort aims to create clear regulatory frameworks for how companies design, validate, and update AI algorithms in medical devices.
Under current rules, most device approvals are “locked” — meaning once software is cleared, it can’t evolve without a new review. But AI models that continually learn pose new challenges:
How do you ensure accuracy after an algorithm updates itself?
How do you verify safety, fairness, and bias?
Who is accountable if AI gives the wrong recommendation?
The upcoming guidance is expected to address:
Algorithm transparency – how manufacturers document how their models make decisions.
Continuous learning oversight – how updates are tested and approved post-market.
Data integrity and bias prevention – ensuring training data represents all populations.
Cybersecurity and privacy protections – keeping AI-connected devices safe from tampering.
The FDA is now collecting feedback from hospitals, manufacturers, and distributors before finalizing its framework — likely by mid-2026.
What This Means for AEDs and Emergency Medical Equipment
AI regulation might sound like it only affects complex hospital systems, but it has major implications for AEDs and field-deployed rescue devices.
Today’s AEDs already use proprietary algorithms to read ECG data and determine whether a shock is advised. As technology advances, those same devices may begin integrating:
Predictive analytics to gauge patient outcomes,
Wireless connectivity to EMS networks, and
Cloud-based data sharing for training and maintenance logs.
At that point, the FDA could classify certain next-generation AEDs as AI-enabled medical devices — meaning they must meet stricter testing and reporting requirements.
For buyers and procurement officers, this means it will soon be important to know whether an AED model complies with evolving FDA AI standards — especially for government agencies, schools, and healthcare facilities where compliance affects liability and funding.
The Opportunity and the Challenge Ahead
Opportunities
AI-enabled equipment can dramatically improve emergency response:
Faster decision-making: Devices that analyze patient data instantly.
Predictive maintenance: Systems that detect wear or calibration issues before failure.
Connected readiness: Real-time AED self-checks that notify facilities or first responders automatically.
Challenges
With innovation comes new responsibility:
Cybersecurity risks — More connectivity means more exposure to hacking or data leaks.
Validation complexity — Proving algorithm accuracy under FDA rules adds cost and time.
Training and transparency — Staff will need to understand AI decision-making to trust it in critical moments.
Distributors and resellers like AEDPRO will play a key role in bridging that gap — ensuring every product sourced, stocked, or sold is backed by compliant manufacturers and up-to-date certifications.
How Facilities Can Prepare Now
Ask manufacturers about AI features – Any algorithmic or data-learning functions should be disclosed and supported with FDA documentation.
Track software updates – Maintain a record of firmware versions and security updates on all AEDs and connected equipment.
Audit your suppliers – Work only with vendors who follow FDA and ISO standards for device software and data protection.
Educate staff – Train employees to understand the benefits and limitations of AI-driven systems.
Stay informed – Follow AEDPRO’s updates as the FDA finalizes its AI regulatory pathway through 2026.
The Smart Future of Life-Saving Equipment
AI is reshaping the landscape of emergency medical response — enabling faster, smarter, and more connected care. As technology evolves, safety and compliance will remain paramount.
AEDPRO is committed to helping our clients stay ahead of these changes by curating FDA-compliant, field-tested equipment and providing the latest updates on evolving regulations.
Whether you’re outfitting a city department, a healthcare facility, or a workplace safety program, our goal is to make sure your equipment — and your people — are always ready.
Stay updated:
➡️ Bookmark our AEDPRO Blog
➡️ Contact us for compliant AED recommendations
➡️ Follow us for 2025 regulatory updates and product releases
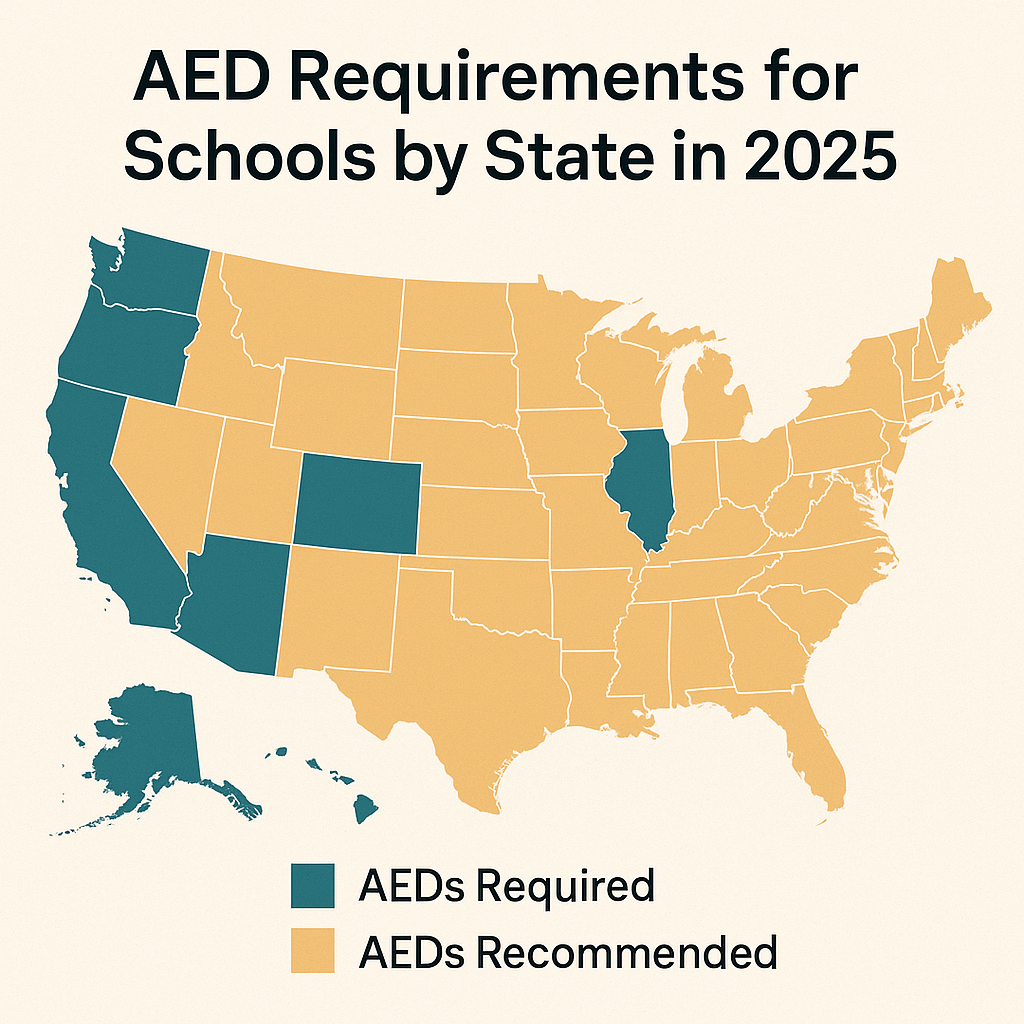
AED Requirements by State in 2025: Updated Compliance Guide for Schools, Gyms, and Businesses
Stay compliant in 2025. Learn AED requirements by state for schools, gyms, offices, and public facilities. Updated laws and guidance to protect lives and avoid fines.
In 2025, many states including Georgia, Texas, Virginia, Massachusetts, and California require AEDs in schools. Laws vary by state, with some covering all K-12 schools and others requiring AEDs at athletic events or campuses only. Most states do not mandate AEDs in all workplaces. However, industries with high risk such as gyms, airlines, and large public venues often face specific AED laws or OSHA-driven recommendations."
Yes. All 50 U.S. states provide some level of immunity for bystanders who use an AED in good faith. These laws are designed to encourage quick action without fear of legal consequences.
Introduction
Sudden cardiac arrest (SCA) strikes without warning—and every minute counts. The American Heart Association estimates survival rates decline 7–10% for every minute a victim goes without defibrillation.¹ Yet, many facilities remain unprepared.
While there is no sweeping federal law mandating AEDs everywhere, states across the U.S. increasingly pass legislation requiring or encouraging AEDs in schools, athletic facilities, workplaces, and public spaces. In 2025, keeping up with evolving state laws is more important than ever—for safety, compliance, and liability protection.
In this guide, we provide a state-by-state snapshot of AED laws (schools, gyms, public access), highlight trends, and share actionable advice to keep your facility ready—whether or not your state mandates an AED.
Why AED Laws Matter in 2025
Life-saving potential. The faster an AED is applied after collapse, the higher the chance of survival.
Liability & legal risk. Failing to provide an AED where required can expose organizations to legal claims.
Public expectation & reputation. Customers, parents, employees, and visitors expect safety measures in modern facilities.
Legislative momentum. New laws, especially in school systems and public venues, are passing regularly (e.g. Georgia’s 2025 requirement for all K-12 schools)
Even in states where AEDs aren’t yet mandated, adopting best practices positions your business or institution ahead of regulation and improves safety.
Federal & National Guidance: OSHA, CDC, and “Good Samaritan” Laws
OSHA & Workplace Programs: OSHA doesn’t mandate AEDs specifically in its standards, but it does encourage organizations to adopt AED programs under first-aid and workplace safety guidelines.
Maintenance & Reporting Requirements: Many states require or encourage routine testing, maintenance, and reporting of AED usage to EMS or state agencies.
Liability Protections (Good Samaritan Laws): All 50 states provide some level of civil immunity for bystanders using an AED in good faith, protecting lay rescuers from liability in most cases.
Taken together, federal and state frameworks encourage readiness, training, and program oversight—even where hard mandates don’t yet exist.
State-by-State Snapshot (2025)
Who Should Install an AED Even If It’s Not Required
Just because your state doesn’t legally require an AED in your facility doesn’t mean you shouldn’t install one. Consider an AED in:
Offices with 50+ employees or high foot traffic
Churches, community centers, and auditoriums
Manufacturing plants, warehouses, or factories
Hotels, shopping centers, event venues
Sports clubs, youth leagues, recreational facilities
Installing an AED proactively improves safety and may give you a competitive or reputational advantage.
How to Stay Compliant & Maintain an AED Program
Even where AEDs are legally required, many organizations fail to remain compliant over time. Use the checklist below to maintain readiness:
Scheduled maintenance & self-tests
Ensure your AED meets manufacturer specs for battery checks, electrode pad expiration, and self-tests. States often require or encourage routine maintenance.AED use reporting & oversight
Some states require that any clinical use of an AED be reported to EMS or medical oversight.Medical direction / oversight
Many AED programs must have a licensed physician or medical authority supervising protocols.Staff training & drills
CPR/AED training for staff (especially first responders) and regular mock drills to test response.Emergency Action Plan (EAP)
Build, document, post, and rehearse your cardiac response plan—include who retrieves the AED, who calls 911, who uses the device, etc.Signage & visibility
Keep AEDs clearly labeled, easily accessible, and known to all occupants.Replace components on schedule
Batteries and electrode pads have limited shelf life—replace before expiration.Annual or semiannual audit
Review every component of your AED program: readiness, logs, staff awareness, signage, medical oversite.
Trends & 2025 Updates to Watch
Expanding school mandates. States like Georgia are broadening school AED requirements to include all K-12 (not just high schools).
Increased CPR/AED Training Laws. New laws (e.g. Texas’s Landon Payton Act) require more school staff to hold current credentials and conduct drills.
Focus on accountability & reporting. Many states now require use-reporting, program audits, and integration with EMS.
Grants & funding push. Federal grants (like via the HEARTS Act) encourage schools and non-profits to adopt AEDs and emergency response plans.
Conclusion & Call to Action
In 2025, being proactive with AED readiness is no longer optional—it’s essential. Whether your state requires an AED or not, a well-implemented AED program elevates safety, protects your organization, and positions you ahead of regulation.
If you’d like help choosing the right AED package, managing maintenance, or designing a full AED program for one or multiple sites, AED Pro is here to help. Contact us for a free compliance consultation and let’s make your facility a safer place.
Why Now Is the Time to Replenish Your AED & Emergency Supply Inventory
Now is the time to stock up on life-saving equipment. The American Hospital Association warns that new tariffs could raise costs on critical medical devices, batteries, gloves, and accessories by 25–50%. (Read more here)
That means waiting could mean:
Higher prices on AED pads, batteries, and accessories
Longer lead times as supply chains tighten
Strained budgets for hospitals, schools, and organizations
At AEDPRO, we recommend reviewing your inventory now:
Check pad and battery expiration dates
Forecast your needs for the next 12–18 months
Order critical items like AED pads and batteries early
We’re here to help you lock in today’s pricing, build a replenishment plan, and make sure your life-saving tools are always ready.
👉 Shop now at AEDPRO and beat the tariffs before they impact your budget.
Reference:
https://www.aha.org/2024-07-01-fact-sheet-impact-tariffs-health-care-equipment
Texas Law Now Requires Choking Rescue Devices in Schools: What You Need to Know About HB 549 and LifeVac
Texas Law HB 549: Choking Rescue Devices Now Required in Schools
AEDPRO and Capitol CPR LLC Are Here to Help with LifeVac Solutions
In a major step toward enhancing student safety, Texas Governor Greg Abbott signed HB 549 into law, mandating that public and charter schools across Texas maintain choking rescue devices on-site. The law, effective September 1, 2025, aims to improve emergency preparedness in schools by requiring tools specifically designed to address choking incidents — a leading cause of accidental death in schools and childcare settings.
Under this new legislation, each school is required to have FDA-registered choking rescue devices available and accessible on campus, and staff must receive proper training in their use.
What Does HB 549 Require?
Effective September 1, 2025, HB 549 requires all Texas public school districts and open-enrollment charter schools to:
Equip each campus with a choking rescue device, such as LifeVac.
Ensure staff are trained in the proper use of these devices.
Make the devices readily accessible in areas where choking incidents are most likely to occur (e.g., cafeterias, gymnasiums, and nurse's offices).
This requirement aligns with ongoing efforts to protect students during emergencies, particularly when time is critical and traditional methods like the Heimlich maneuver are unsuccessful or cannot be administered.
Why LifeVac?
Among the most trusted choking rescue devices on the market is LifeVac, an FDA-registered, non-invasive airway clearance device designed to help save lives in choking emergencies. LifeVac is easy to use, requires no power source, and has already been credited with saving hundreds of lives across the country.
At AEDPRO, we are proud to be a licensed distributor of LifeVac, offering:
✅ Best-in-class pricing for schools and institutions
✅ Training support to meet compliance and confidence standards
✅ Fast shipping and bulk ordering options
📦 Order LifeVac now to stay ahead of HB 549 compliance:
👉 https://www.aedpro.shop/shop/shop-by-brand/lifevac
Our Commitment to Texas Schools
At AEDPRO and our partner company Capitol CPR LLC, our mission is to empower schools, businesses, and communities with the tools and training they need to respond to emergencies. With HB 549, Texas has raised the bar for student safety — and we’re here to help you meet that standard with confidence and ease.
We’re not just suppliers; we’re partners in readiness
Attention Texas parents, teachers, school administrators, and childcare professionals — a new law is making waves in school safety, and it’s all about saving lives when every second counts.
As of September 1, 2025, Texas House Bill 549 (HB 549) mandates that public schools and open-enrollment charter schools must have choking rescue devices on-site. This landmark legislation is a crucial step toward improving emergency preparedness and ensuring our children are protected from one of the most common and silent threats in schools — choking.
At AEDPRO, we’re proud to support this life-saving initiative by being a licensed distributor of LifeVac, the industry-leading choking rescue device that is FDA-registered and easy to use by non-medical professionals. We also provide comprehensive training to ensure your staff is confident and ready to respond.
What Is Texas HB 549?
Texas HB 549 was signed into law to require choking rescue devices, like LifeVac, in schools across the state. The law aims to prevent choking fatalities by ensuring that:
Every school campus has at least one choking rescue device in a readily accessible location.
School personnel receive training on how to use these devices properly.
Devices are included in school emergency preparedness protocols, just like AEDs and EpiPens.
This law is a direct response to tragic incidents where students have lost their lives to choking—an emergency that can escalate in mere seconds. With a choking rescue device on hand, those situations can often be reversed quickly and effectively.
Why LifeVac Is the Gold Standard in Choking Rescue
At AEDPRO, we proudly offer LifeVac, the top-rated, FDA-registered anti-choking device that has already saved hundreds of lives worldwide, including children in schools and daycare centers.
Key Benefits of LifeVac:
Non-invasive and easy to use
No prescription required
Works in seconds to clear airway blockages
Comes with masks for children and adults
Proven safe and effective, with real-life success stories
Whether you're equipping a school, daycare, or home, LifeVac is the trusted solution recommended by safety experts and medical professionals.
AEDPRO: Your Partner in Safety and Compliance
As a licensed LifeVac distributor, AEDPRO is here to help Texas schools and childcare centers meet the HB 549 requirements quickly and confidently.
We offer:
✅ Direct purchase of LifeVac devices from our official shop
✅ Group pricing and bulk order options for schools and districts
✅ On-site or virtual training for staff on how to use LifeVac properly
✅ Expert guidance to ensure your facility is fully compliant
Get Compliant with HB 549 Today
Don’t wait until the deadline. Equip your school or daycare with LifeVac now and ensure your staff are trained and ready.
🔗 Shop LifeVac Today: https://www.aedpro.shop/shop/shop-by-brand/lifevac
About AEDPRO
AEDPRO is a trusted, Texas-based distributor of medical and emergency preparedness equipment; a Capitol CPR LLC owned company.
We’re proud to serve schools, parents, and professionals across the state with products that save lives – including AEDs, first aid kits, and the LifeVac choking rescue device.
Stay Safe. Stay Prepared. Choose AEDPRO.